How does physical water treatment work?
How does physical water treatment work?
A VERY detailed article about the science and technology behind physical water treatment, by Dr.-Ing. Hartmut Jünke
1. Introduction
The physical water treatment has been used and discussed for the last two decades. During this time, it has proven its effectiveness that on the other hand is still questioned and denied. Why is that? If we follow the discussions, we can find various reasons that however are not going to be discussed here. It rather seems necessary to examine the physical foundations that can explain the mode of action of these processes and so to free them from the reproach of fraud and to recognize the black sheep that led to this reproach. The following is a try to clear these questions.Apart from my own positive experience that clearly demonstrates the effectiveness, at least of the device installed on my pipes, there is a number of information coming from renowned institutions such as e.g. the Physiological Institute of the Ludwig- Maximilian-University Munich confirming the same. In this institution, the replacement of laser tubes because of furring through the coolant that had been necessary before, could be avoided after the installation of a physical water treatment device (
Hotels and instruction companies as well as a lot of conversations with private users confirm the action, although the non- functioning is also often lamented. As in most cases the private users do not know the producer of the device (a lot of times it was said that the product was a cheap one bought in a superstore), we can only draw the conclusion that there are some devices that do not meet the physical conditions to be effective. But we cannot draw the conclusion that the treatment principle itself is useless and does not work.
Unfortunately, this impression is also often given in serious publications, a lot of times without giving any scientific proof or any proof orientated towards the action and that does justice to it. Before the action of the physical water treatment is explained in a plausibility proof, first we have to clarify why water pipes fur up. Therefore we see the lime as target of the physical water treatment.
2. The Lime
Chemically speaking, lime is calcium carbonate (CaCO3). This compound is not soluble in water.Question: How can it be dissolved in the water then?
Answer: When water that contains carbon dioxide passes chalky grounds, lime is released and is present in the water as calcium hydrogen carbonate Ca(HCO3)2. This is possible as carbon dioxide CO2 together with water H2O forms carbon acid H2CO3. As everybody knows from the everyday household life, acidic cleaning agents are needed to remove lime deposits. It seems like splitting hairs to underline the difference between dissolved and undissolved lime, but this is exactly where the lack of argumentation in favor of the action of these devices lies.
Thereupon the following question is raised: why does lime separate anyway?
The dissolved amount of calcium hydrogen carbonate in the drinking water never reaches the saturation limit that if exceeded leads to the separation of the dissolved substance as a crystal.
If we look at the points in the pipes where lime deposits, the answer is already given. Primary spots for lime deposits are pipe bends, branches and the ending points (faucets) and especially the warm water areas. In the last mentioned case we have to differentiate: warm water containers are generally speaking free from deposits; heating bars, heater spirals or heat exchangers, surfaces that transmit the heat to the water, are always affected.
Why these spots?
The answer is pretty easy: there has to be an energy gradient that leads to the opening of the water cages around the dissolved ions so that they can react with each other. At the same time the so called lime -carbonic acid- balance has to be disturbed, this means that it has to come to a local lack of CO2. Then the elements look for a crystallization point (nucleus) where to start the crystallization. These spots are always located on the walls of the pipes, these represent the solid base on which the crystals can grow. More and more elements deposit, the lime deposits grow and incrustations, also known as scale, develop. They consist of calcium carbonate mixed with magnesium compounds, gypsum, silicates and iron compounds (therefore the yellow brownish color). These sedimentations favor corrosion and worsen the heat transmission of heating bars and heat exchangers.
How is it possible that there are local energy differences in the water?
In the case of heating bars it is easy, heat is transmitted to the water. In pipe bends the water is accelerated, the energy for this process comes from the inner energy of the water, pressure and temperature changes are the consequences. The same goes for branches and ending points. Here, turbulences are caused, also with the inner energy of the water and with the same consequences.
If we take a look at pipes that have been used over years, we can see that incrustations always start in pipe bends or branches and from there grow into the straight areas. When a pipe clogs up, the affected areas are normally these areas, while the predominant part of the pipe system is still completely in working order and able to let the water flow through.
What happens chemically during the crystallization? The following formula (1) explains it:
Ca(HCO3)2 ↔ CaCO3 + H2O + CO2 (1)
In the first place it is remarkable that the described reactions towards a lime formation can also take place the other way around, i.e. the lime can also dissolve again (see above). Which of the two reactions takes place depends on the lime- carbonic acid- balance. If there is a surplus of CO2, lime is dissolved, if not, lime is secreted. These processes are also dependent on pressure and temperature changes, therefore on physical parameters.
At this point, it is appropriate to say something about the lime crystal. It is known that almost all substances defined as solid are crystalline. Crystals are divided in 7 crystal systems and 32 crystal classes, which differ from each other through their lattice structure.
Lime can crystallize in two different structures which are chemically completely identical. The lattice structures are different but related. Afterwards, the lattice type Aragonite (picture 1) or Calcite (picture 2) is formed. When the chemical structure is the same, it depends on thermodynamic circumstances (pressure, temperature) which modification is produced. As the pictures show, in both unit cells, one axis is longer than the others. This means that a crystal grows faster in this direction than in the others. The grow velocity is anisotropic, i.e. dependent on the direction.
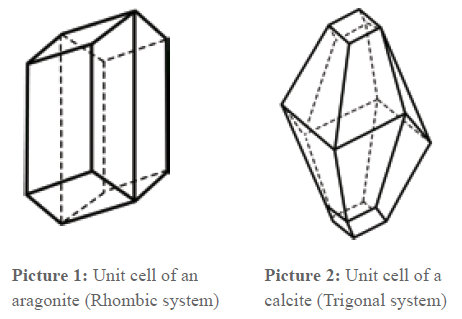
That means that crystals that grow undisturbed develop a needle-shaped form. If the grow velocity was the same in all axis directions, globular crystals would develop. In the lattice type Calcite, there is also a crystallization of magnesium carbonate MgCO3 and FeCO3, and that is why these substances are also incorporated in the scale formation. On the other side, Anhydrite (dried gypsum or gypsum [CaSO4·2H2O]) corresponds to the lattice type Calcite. In similar lattice types phosphates and sulphates such as silicates of calcium and magnesium also crystallize. This favors their incorporation in the deposits. Also for them present crystallization nuclei serve as a starting point for a segregation in the water and not for a deposit on the walls of the pipes or on heating bars, especially in warm water – in which these water companions often dissolve first.
So what do devices do when they show the promised effects?
1. They do not convert lime into what should they? The devices cause that Calcium hydrogen carbonate Ca(HCO3)2 turns out as Calcium carbonate CaCO3, which is electrically and chemically neutral in water. And this is a solid with the special concomitant that the crystal does not crystallize on an already existing solid but is formed in the flowing water. Such a crystal forms according to laws of nature with typical parameters valid for every substance and it takes on a form according to the law of nature.
2. The result is that these crystals do not have special characteristics but special forms that do not attach to each other anymore and therefore prevent calcification. At this point the described mechanism is effective.
3. Water
To understand the following processes, now some information about water is given. It is way more than what the formula H2O says. The two hydrogen atoms and the oxygen atom form an equilateral triangle and incircle a ~110° angle, as shown in picture 3.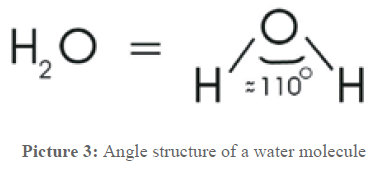
This is the reason for a lot of characteristics that distinguish water from other, similar molecules. Two gases that react with each other form a liquid and not a gas, as it is e.g. with carbon dioxide CO2 (solid substance and gas!), a molecule a lot heavier. Because of this angle position water molecules form chains and clusters that cause the fluid state.
This is possibly the reason why water may have a “memory” in which it adopts structures in the chains and clusters that do not change even when the water moves. These chains and clusters are held together by Van de Waals powers or dispersion powers or hydrogen bridges. The bond is based on the attraction of electric dipoles present in molecules with polarized bonds or angled structure. At the University of Stuttgart, scientific researches are conducted concerning this problem and first results show that the behavior of water is influenced by electric and magnetic fields. Such phenomena have been known for a long time but have never been investigated scientifically.
This molecule form leads to a further special characteristic. Water shows a dipole character. Through the bond, both elements strive for an inert gas configuration in their outer electrons shells. In the case of hydrogen there are two electrons, in the case of oxygen eight. Oxygen is missing two and each hydrogen one electron. In the molecule the total of two bonding electrons is available for all three atoms, so that an inert gas configuration can be reached by all molecules.
In all homopolar bonds of diverse atoms, the bond is polarized, i.e. the bonding electrons pair is moved towards the direction of the bonding partner with the higher electron affinity, in this case the oxygen atom. If the water molecule is put in an electric field, it lines up so that the oxygen shows towards the positive electric side and the hydrogen molecules towards the negative electric side. So the water molecule is charged a little bit more negatively on the side of the oxygen and a little bit more positively on the side of the hydrogen.
This fact, together with the molecule form, plays an important role for the dissolving ability of water and for the physical water treatment. At this point further anomalies are only briefly mentioned: when water passes to a solid state (ice) its density decreases. If the ice is put under pressure, it liquefies again. Normally, liquids under pressure pass to a solid, crystalline state. These few indication already show that there is probably a lot more about water than today’s modern research has discovered so far.
4. Physics and Chemistry
What happens physically and chemically when a physical water treatment device is used?
As there are a lot of different application principles, from magnets introduced in the water pipes to the injection of seed crystals in the water, around which lime particles attach, in the following only one principle often offered and often controversially discussed is going to be examined.
The processes are described on the basis of a device with an appearance often found and which effects are questioned. Picture 4 represents this device. The test explained here is based on the functioning and mode of action of this device. It is a blackbox from which two cables exit and are wound around the pipe. These cables transmit oscillations to the water that are supposed to “convert” the dissolved lime and render it harmless.
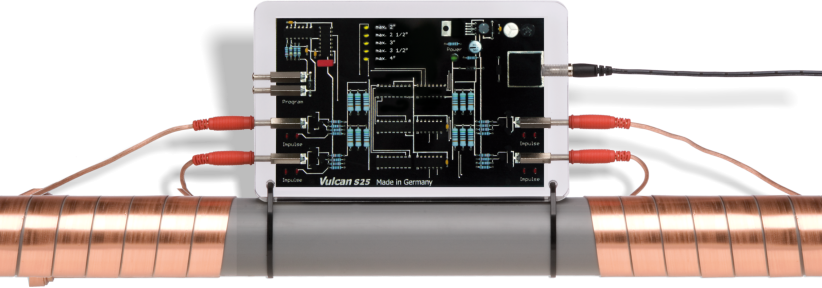
This formulation has been chosen intentionally because it essentially corresponds to the description of the function of offered devices and therefore already puts in doubt the repute and seriousness.
What kind of oscillations are transmitted?
Some descriptions do not even talk about calcium being converted; the producers seem to come from the times of the al alchemist. Some say that the pipe material does not matter and that the device can even remove already existing lime incrustations.
How can oscillations achieve all this?
Seriously, who thinks to understand just a little bit of physics and chemistry already finds enough apparently scientific arguments to question the functioning.
What does a device do that really prevents lime deposits in pipes? At this point the first question has to be what it has to do to fulfil this demand?
The answer is easy: It has to create the conditions under which the calcium hydrogen carbonate Ca(HCO3)2 is washed away with the water as a crystal and does not attach to the pipe walls as calcium carbonate crystal CaCO3.
In the following, the physical and electrical possibilities that an effective physical water treatment system has to offer are examined. This simply means that it has to cause the effect that the dissolved lime does not attach in crystalline form to the walls or contact points with the pipes, to devices and fittings in contact with water. This is only possible if the dissolved lime crystallizes in the water before the contact with these areas.
Therefore two conditions in the water have to be fulfilled:
1. Crystallization nuclei have to be present or created.
2. The lime- carbonic acid- balance has to be changed so that dissolved lime becomes solid.
Experience has shown that the introduction of magnetic or electric fields in the water can have such effects, even if with different success. In the following, only the effects of electric fields are examined, but from these the conditions under which magnetic fields can also be effective can be derived.
If we take a look at picture 4, we can see the two windings through which impulses are transmitted. A lot of producers call these windings “coils” because they look like coils, but electrically speaking they are not. So an “inductive” coupling is not possible and if it was inductance, the device would fail in the case of iron pipes, but it does not. The winding represents a part of a capacity, it is one capacitor surface, the other one is the water. This winding is a technological compromise; a metal foil placed around the pipe on the same length would have a slightly higher capacity, but would also have to be custom made for every pipe diameter. Normal loudspeaker cables instead are sold in meters and adapt to the different pipe diameters without any problems.
How can, with this arrangement, an electric field be caused in the water even through every pipe material?
This is the point where most doubts begin. With this arrangement, it comes to a physical effect widely spread in the electric everyday life but not well known: the influence. In picture 5 the principle of the process is shown on the basis of a capacitor.
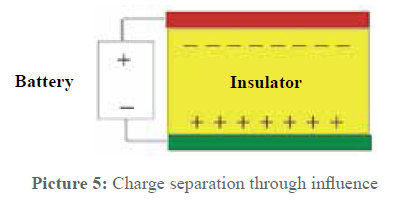
When voltage is transmitted to the two capacitor plates, a charge displacement in the dielectric (insulator) is caused, which is the opposite of the charge of the plates. When the plates are discharged, the polarisation of the insulator also disappears as in the insulator electrons cannot move but only bound electrons are displaced.
But if on the other hand e.g. two metal sheets laid on top of each other (electric conductor) are put into the electric field between the capacitor plates, the charge separation is the following: the surface of one metal sheet gets a negative charge (opposite of the positive capacitor plate) and the other one gets an equivalent negative charge. This phenomenon is called influence. If the two plates in the electrical field are separated, one of the plates shows a negative charge (surplus of electrons) and the other one a positive charge (shortage of electrons). A capacitor is impermeable for direct voltage but not for alternating voltage. This fact is used when it comes to introduce electric alternating fields in the pipes. Picture 6 is an instant photograph of this process. You can see that the pipe material does not have any influence on the capacitor effect in the arrangement.
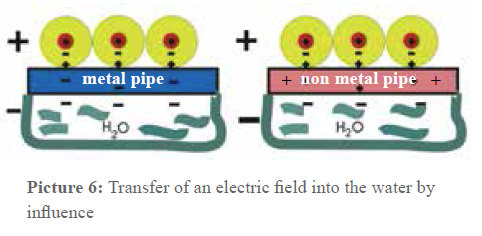
If the winding wire is charged by a pole of a power source, the same electric charge of the opposite sign is bound in the water pipe through influence (by flow from the earth). If it is a temporal periodic charge transfer, or, respectively, a charge and discharge, a so-called displacement current is produced - like in a capacitor (apparently) influenced by alternating current - between the insulated winding wire and the pipe wall (this can be calculated with the Maxwell equation).This is the continuation of an alternating (+-+-+-…) or pulsating (0+0+0+0… or 0-0-0-…) conduction current which develops between the pipe (including the water) and the ground.
This results on the one hand from an alternating or pulsating electric field orientated in the longitudinal direction of the pipe and on the other hand from a magnetic eddy field centrically wound around the pipe. Measurements have shown that an effective voltage of ≈1 Volt is produced between the winding and the water and that there is a displacement current of ≤ 5μA.
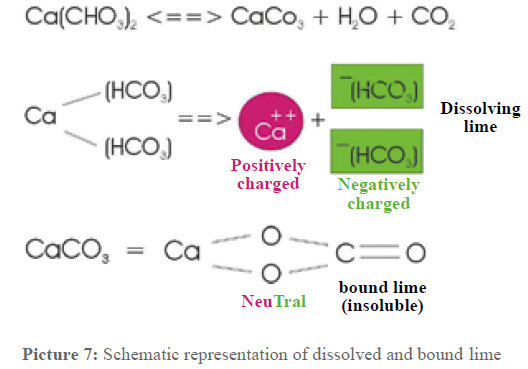
At this point, some more attention has to be paid to the lime dissolved in the water. Picture 7 shows the connections. The dissolved lime – calcium hydrogen carbonate – dissociates in one double positively charged calcium ion and two negatively charged hydrogen carbonate ions. These ions are surrounded by a water cage. The water molecules settle around the calcium so that the oxygen points towards the calcium and the hydrogen towards the outside. Electrostatic powers hold these clusters together. The carbonate remnants are surrounded in the same way only that the oxygen atoms of the water molecules point to the outside. These clusters altogether show a positive or respectively negative charge.
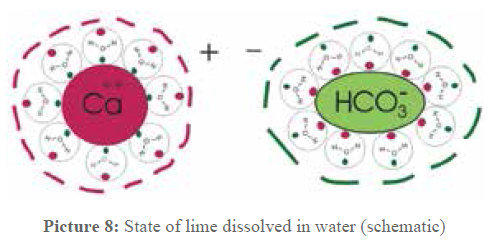
A schematic representation is given in picture 8, the clusters just have to be imagined as minute spheres. They have a diameter of 1 to 2 nanometers (nm), assuming that about 100 to 200 water molecules are involved. If the mass of these clusters is calculated, it results that the mass of the Ca- clusters as well as the mass of the bicarbonate remnants is of 30×10-22g up to 60×10-22 g. These results are interesting for the water treatment.
Coming back to the inducted electric alternating field, it is to say that that the periodically alternating field in the inside of the pipe influences the ions or dipolar molecules closed in water cages in the water in a way that they move from the one direction of the pipe to the other to the beat of the alternating field. The electric oscillation has led to an oscillation of matter which spreads axially. Physically, this is a mechanical (acoustic) longitudinal wave or shock wave. Areas with overpressure and negative pressure alternate. In atomic and molecular fields, this locally causes an adhering of the CO2. If the oscillation frequency is suitable, the water cages disintegrate and this also leads to a local decrease of the CO2 concentration. The lime- carbonic acid balance is locally disturbed and at the same time the dissolved lime ions that are freed from the water cage can meet and react with each other: a lime molecule has been produced which now serves as crystallization nucleus.*) Other molecules are taken up by this nucleus and form a lime crystal in the water. This lime crystal is electronically neutral and does not react in tap water any more. Therefore, this lime crystal is not taken up by existing lime deposits on the pipe walls anymore.
To cause these processes, the electric alternating field has to contain frequencies that if possible lead to resonance oscillations of the water cages. Since all tap waters that correspond to the German drinking water decree are different regarding the quantity of dissolved minerals, the pH-value and the conductivity, the formation of the electric alternating field is also influenced. Besides, there is the changing flow velocity. Devices that work with only one frequency can also successfully set off this cycle by chance, but most of the times they do not show any success.
A couple of technical data about the device examined here are known as well as positive experiences about the effect. Therefore, it makes sense to theoretically and (as far as possible) practically assess the effectiveness of the device by means of these information.
The device is provided with two windings. Each winding receives impulses with a clock frequency of 10 Hz, 50 ms pulse duration, 50 ms rest and de- energize. When one has the rest and de-energize, the other one receives the impulses. Each impulse has a frequency response of ca. 3 to 15 kHz, spread on 50 ms. As there was no suitable measuring technique, the frequency response could not be measured. If 10 oscillations are counted per kHz, the pulse duration is approximately reached. At this point it has to be particularly emphasized one more time that this is only an attempt to generally explain the effectiveness. The complexity of the excited oscillations including the overlapping of different waveforms (overtones) cannot be taken into consideration.
The device is supposed to safely treat 5000 liters of water per hour. In the case of a half inch pipe this means the flow of a water column of 11.3 mm/ms, in the case of a one inch pipe it would be 2.8 mm/ ms and in the case of a two inch pipe 0.7 mm/ms. As the length of the effect of an electric alternating field is of ≈ 500 mm (the producer indicates ≈ 1000 mm), this means that this distance is just covered. Every ion water cage has enough time to fall apart.
What about the reaction velocity of the chemical components? The Max-Born-Institute for nonlinear optics and transient spectroscopy in Berlin has examined the velocity of the formation of molecules on the basis of water molecules with a special laser array. The result was a time between 10 and 20 femto seconds (1 fs = 10-15 second). This time is as inconceivably short as the universe is inconceivably big. The distance light travels in 1 fs gives us an approximate idea of how short this time is: ≈ 0.3 μm. In the time light travels 6 mm, 1000 molecules can be formed. Therefore, it is very probable that the molecule formation and the formation of nucleus crystals take place in the section treated.
5. Protective layers and incrustations
At this point the formation of incrustations is only briefly mentioned for the processes in pipe bends. The flowing water accelerates in the pipe bends. The water flowing in the outer radius is faster than the water in the inner radius. According to the simplified Bernoulli’s equation (2), the sum of the static and the dynamic pressure is constant:Pdyn + Pstat = const. (2)
In the water that flows faster, the dynamic pressure increases and the static pressure decreases. This means that CO2 escapes from the inner radius towards the outer radius and the lime- carbonic acid- balance is disturbed. Lime is set free, looks for a crystallization point and finds this point on the walls of the inner radius. Little by little, a layer of lime epitaxially grows in which other minerals also deposit. On this irregular surface turbulences develop, the same happens in pipe branches because of pressure fluctuations, so that in both cases lime deposits develop.
As water, and therefore also the CO2, evaporates in faucets and shower heads, also here lime deposits develop. On heated surfaces the CO2 is also removed from the closer surrounding area, so that theses surfaces are also favorite crystallization points for the lime.
For two reasons, the presence of lime in drinking water is important and therefore a minimal amount that corresponds to a water hardness of 8.4°d is stipulated by the German drinking water decree. Firstly, the drinking water provides a big part of calcium the body needs and secondly, the bicarbonate remnants of the dissolved lime reacts with the metal of the pipe and so forms a metal carbonate protective layer. This is especially important in the case of copper pipes (see below). Picture 9 shows a detail of such a protection layer. You can see how the crystals grow on the metal surface. Such bundles of crystals cover the surface and protect the pipe against corrosion.
Picture 10 shows this even better. It is an electron microscopic picture of an artificially produced phosphate protective layer against corrosion. Phosphates crystallize in a similar crystal system as carbonates. In time, this desirable quality of lime becomes a disadvantage as more and more lime deposits grow in these protective layers since they are ideal crystallization points. Slowly, a pipe clogs up, starting in the pipe bends and branches.
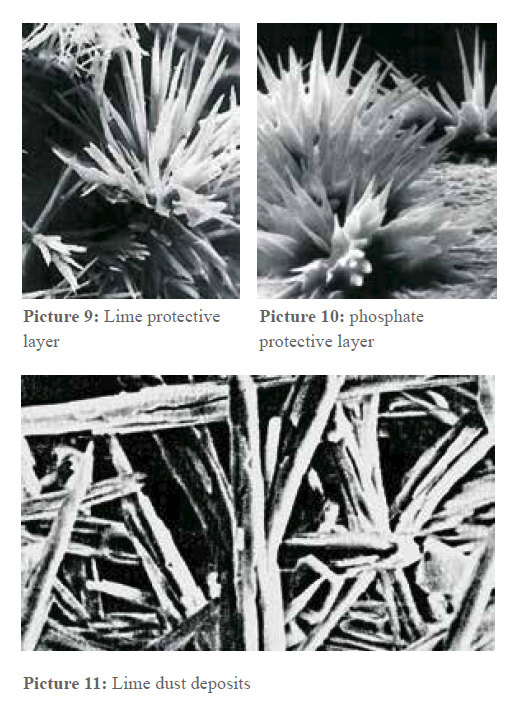 As indicated above, from here the incrustations grow into the straight sections of the pipe. This process takes places as long as there is dissolved lime in the water. But most of the lime transported in the water is washed out of the pipe without depositing. After all, with a water consumption of 100 m³ per year and a water hardness of 28°d, about 45 kg of lime are transported through the pipes. If the lime has been transformed into crystals in the water as described above, the lime is washed out of the pipe with the water in form of a fine submicroscopic crystal, a crystallization on the walls of the pipes is not possible anymore. The lime crystals deposit irregularly, as shown in picture 11.
As indicated above, from here the incrustations grow into the straight sections of the pipe. This process takes places as long as there is dissolved lime in the water. But most of the lime transported in the water is washed out of the pipe without depositing. After all, with a water consumption of 100 m³ per year and a water hardness of 28°d, about 45 kg of lime are transported through the pipes. If the lime has been transformed into crystals in the water as described above, the lime is washed out of the pipe with the water in form of a fine submicroscopic crystal, a crystallization on the walls of the pipes is not possible anymore. The lime crystals deposit irregularly, as shown in picture 11. This condition stays the same also in warm water. Applications have shown that further dissolved minerals deposit on the nuclei build o the lime crystals and sink to the bottom of e.g. water boilers in form of dust without growfing on the heating bars. This way, 2 kg of lime dust deposits could be removed from a 150 liter water boiler after a year of operation, the heating bars were absolutely scale free. User report that the heat exchangers for the hot-water supply in the case of district heating also stay lime free on the secondary side. Since the installation of the examined device four years ago, no cleaning has been necessary. The lime has been made harmless but has not been removed and is still physiologically present. Another consequence of this is that water drops that dry on surfaces leave lime dust which can be removed with a humid cloth. But if it is left in a humid surrounding for a while, it can locally dissolve under the influence of the CO2 in the air and if it dries again, a crystallization on the surface is possible: This incrustation can only be removed with a decalcifier.
But these devices are also supposed to remove existing deposits and to prevent rust or corrosion. Is this possible? And if it is possible, how does it work?
6. Removal of deposits and protection against corrosion
First of all, some information about the removal of lime deposits: If we take a closer look at the equation (1) we can see that the chemical reaction can not only take place from the left to right side (lime segregation) but also from the right to the left (lime dissolution). Here again, the lime- carbonic acid balance plays a crucial role. If there is a surplus of carbonic acid, lime is dissolved. With each dissolved lime molecule crystallized in the water one carbonic acid molecule is produced. This carbonic acid gradually attacks and dissolves the lime deposits on the pipe walls and so removes the lime. Depending on the level of the incrustations in the pipe (water hardness, working life), this process can take between half a year and two years. During this time, light lime deposits outside the water develop again. When this process is finished, no more incrustations develop. The lime is removed, but the carbonate protective layer is maintained.Of course the lime crystal in the water is also exposed to this influence. But the crystal produced in the water has been able develop in an almost weightless state and therefore a crystal structure forms that shows only a few lattice defects such as vacancies, interstitial atoms, substitution atoms and molecules, displacement and stacking faults. Therefore, this crystal offers less targets than the incrustation presenting these errors and therefore also a bigger surface and with that a higher inner energy. This is why this incrustation is attacked more, often with a selective dissolution, what leads to the eruption of coarser lime particles which can accumulate in the aerators.
Now the equation (1) represents a balanced stationary state. But in nature fixed equilibria do not exist, only flowing equilibria. At the melting point of water e.g. ice and water exist at the same time, therefore balanced. This means that statistically in one time unit the same amount of water molecules changes from the liquid to the solid state as water molecules melt from the ice.
The equilibrium is flowing. The lime segregation as well as the lime dissolution described in the equation (1) is also subject to this static process, if there is no intervention from the outside. The processes in the section treated will not catch all present molecules. Even if in smaller amounts, there will still be dissolved lime in the pipe which can also segregate but then be dissolved again. But since the physical water treatment intervenes in favor of the dissolution of the lime and the removal of the deposits, new incrustations do not form. Statistically, it is possible that during these processes surfaces that are not yet covered with metal carbonate crystals (see pictures 9 and 10) now form such crystals and so make the corrosion protection layer thicker.
The described mechanism of the formation of a protective layer is not the only effect preventing corrosion. Since there is already a protective layer, normally no corrosion should occur, but as experiences show corrosion does occur, in galvanized iron pipes as well as in copper pipes. What is the reason?
In technology there is a corrosion process called ventilation element. Picture 12 describes this process. Iron is an electric conductor, water is an electrolyte. When a water drop lies on the iron, an electrolytic element has been formed; the only thing missing is the electric voltage. At the edge of the water drop the oxygen contact towards the metal surface is stronger; the center of the drop is less ventilated. Thus a potential difference between these two areas develops; the edge of the water drop becomes a cathode (surplus of electrons) and the center of the drop an anode (shortage of electrons). Being an electrolyte, the water now enables the closed electrical circuit between anode and cathode. At the anode, positively charged ions of the prevailing metal dissolve, react with the water and deposit as rust, while the electrons take the way through the metal to the cathode. In principle, the process is the same in the case of copper.
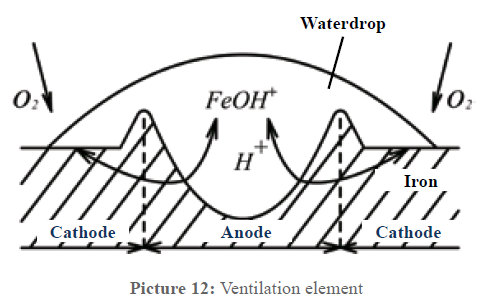
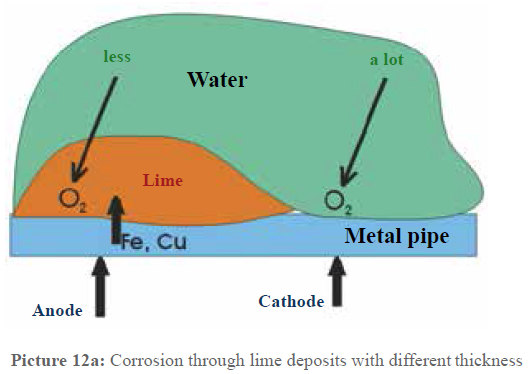
In principle, the same process takes places in our pipes, the only difference lies in the reasons for the different oxygen contacts to metallic surfaces. Picture 12a schematically represents this constellation. As long as the water is not physically treated, lime favors to deposit, as described above. Between the areas with strong lime deposits and the lime free areas, this causes that the more or less strong oxygen contact in the water effects the surfaces with a different concentration. This way, the same process as in the ventilation element is caused. As it is generally known, most of the times corrosion occurs in pipe bend branches and T-pieces which show thick deposits. If these deposits are removed leaving only the protective layer, the oxygen contact is the same everywhere and an electric potential cannot develop anymore. This process is especially important for copper pipes, as with a high oxygen content and pH values lower than 6.5 copper is especially corrosion endangered and specially tends to pitting corrosion. In these cases a thick protective layer is especially important, also because of the impurity of the copper (supplier of cheap products) that favors the formation of local elements. Thus, more and more copper comes into the water and this is unfavorable for the health. According to the recommendations of the Federal Ministry of health, babies should not drink tap water in these cases. Water suppliers call copper the “lead of the 20th century”.
7. Closing Remarks
The dealt facts show that the effectiveness of the physical water treatment has not only been proven by users but that there are also theoretical and practical physical-chemical proofs. But a precondition is that the device offered more or less fulfils the described parameters. In general, the electronictechnological demand is pretty high, so that most of the times cheap devices cannot fulfil the demands. The mode of action of these devices shows that the usual test procedures to determine the effectiveness, especially short tests have to fail and provide false results. A new testing procedure has to be developed which can also provide a quantitative proof of the theoretic connections described here.
I would like to thank Prof. Dr. H. Ungenannt, Mageburg, for the support during the interpretation of the electric processes, Mr. K. Matthies, Dipl.-Ing., Berlin, for the help concerning the measurement technology, Prof. Dr. W. Morgner, Eichenbarleben, for the critical discussions of the present work and the engineering firm for physical water treatment Helmut Siegmund, Königs-Wusterhausen, for the provision of the device.
(1) Information about the device can be sought over the manufacturer — Christiani WassertechnikGmbH, Köpenicker Str. 154, 10997 Berlin, Germany.
Photo credit:
• Pictures 1 and 2: W. Kleber, Einführung in die Kristallographie, Verlag Technik Berlin• Picture 4, 6, 9 and 11: Information script from Christiani Wassertechnik GmbH
• Picture 10: Information script from the BMW motorcycle factory Berlin
• Picture 12: W. Schatt (editor), Einführung in die Werkstoffwissenschaft, VEB Deutsche Verlag für Grundstoffindustrie, Leipzig
*) Coral animals build their coral sticks on the same basis. In their feet area, they have plant cells that contain chlorophyll. This produces organic material (carbohydrates) from water and CO2 by the means of sunlight. Thus, the lime- carbonic acid- balance is also disturbed (reduction of the CO2) and this leads to a secretion of lime forming the coral sticks. This is a reason why corals only exist in sun flooded shallow water, as only here there is enough sun energy for the photosynthesis process.